Osmosis and Tonicity
RicochetScience・2 minutes read
Osmosis involves the movement of water across a semi-permeable membrane influenced by tonicity, which examines solute concentrations in different environments, significantly affecting red blood cells' behavior in various solutions. Understanding these processes is essential for kidney function and managing conditions such as diabetes, as they dictate cell hydration and integrity in response to different osmotic conditions.
Insights
- Osmosis involves the movement of water through a semi-permeable membrane, and the concept of tonicity helps explain how different solute concentrations affect red blood cells; for instance, when these cells are in pure water, they can swell and burst, while in a salt solution, they lose water and shrink. Understanding these processes is essential not only for grasping basic biology but also for recognizing their significance in kidney function and conditions such as diabetes, where osmotic balance plays a vital role in health.
Get key ideas from YouTube videos. It’s free
Recent questions
What is osmosis in simple terms?
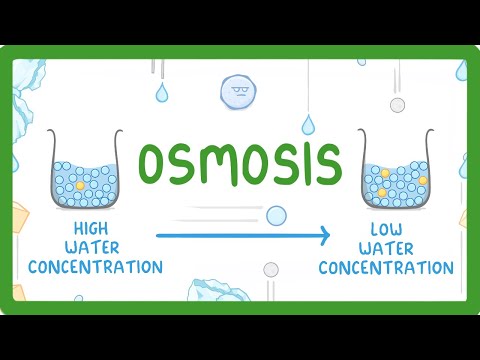
Osmosis is the process where water moves through a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration. This movement continues until there is an equal concentration of solutes on both sides of the membrane. It is a vital biological process that helps maintain cell turgor and overall fluid balance in organisms. Understanding osmosis is essential for grasping how cells interact with their environment, particularly in terms of nutrient absorption and waste removal.
How does tonicity affect cells?
Tonicity refers to the relative concentration of solutes in a solution compared to another solution, particularly in relation to cells. It affects how water moves in and out of cells, which can lead to different outcomes based on the type of solution the cells are placed in. In a hypotonic solution, cells may swell and potentially burst due to water influx, while in a hypertonic solution, cells can shrink as water exits. Isotonic solutions maintain cell size as there is no net movement of water. Understanding tonicity is crucial for various medical and biological applications, including intravenous therapy and cell culture.
What happens to red blood cells in water?
When red blood cells are placed in pure water, which is a hypotonic solution, water enters the cells because the inside of the cells has a higher solute concentration. This influx of water can cause the cells to swell and potentially burst, a process known as lysis. This phenomenon highlights the importance of maintaining proper osmotic balance in the body, as excessive swelling of red blood cells can lead to serious health issues. It also underscores the need for careful management of fluid intake and electrolyte balance in medical settings.
Why is osmotic pressure important?
Osmotic pressure is the pressure required to prevent the flow of water across a semi-permeable membrane due to osmosis. It is a critical factor in maintaining fluid balance within cells and tissues. Osmotic pressure drives the movement of water from areas of lower solute concentration to areas of higher solute concentration, which is essential for processes such as nutrient absorption and waste removal. In the kidneys, osmotic pressure plays a key role in filtering blood and regulating body fluids, making it vital for overall health and homeostasis.
How does diabetes relate to osmosis?
Diabetes affects the body's ability to regulate blood sugar levels, which can lead to changes in osmotic balance. High levels of glucose in the blood create a hypertonic environment, causing water to move out of cells, leading to dehydration and increased thirst. This osmotic imbalance can also affect kidney function, as the kidneys work to filter excess glucose and maintain fluid balance. Understanding the relationship between diabetes and osmosis is crucial for managing the condition and preventing complications related to fluid and electrolyte imbalances.
Related videos
Cognito
GCSE Biology - Osmosis #8

Khan Academy
Hypotonic, isotonic, and hypertonic solutions (tonicity) | Khan Academy

BOGObiology
Hypertonic, Hypotonic and Isotonic Solutions!

CrashCourse
In Da Club - Membranes & Transport: Crash Course Biology #5

Osmosis from Elsevier
Diabetes mellitus (type 1, type 2) & diabetic ketoacidosis (DKA)
Summary
00:00
Osmosis and Tonicity in Cell Dynamics
- Osmosis is the diffusion of water across a semi-permeable membrane, while tonicity refers to the relative solute concentration of two environments separated by such a membrane; when red blood cells are placed in pure water (hypotonic solution), water enters the cells (which are hypertonic) causing them to potentially burst (lysis), whereas placing them in a hypertonic salt solution (like sodium chloride) causes water to leave the cells, leading to cell shrinkage (crenation), and in an isotonic solution, there is no net water movement, with osmotic pressure always driving water from hypotonic to hypertonic environments, which is crucial for kidney function and understanding conditions like diabetes.




