NEET 2025: Chemical Kinetics | Complete Chapter | Part 1 | Wasim Bhatt
Unacademy NEET English・68 minutes read
The chapter covers chemical kinetics basics like concentration changes, reaction rates, and moles of products and reactants over time, essential for understanding chemical reactions. It explores concepts like average rate of reaction graphically and mathematically, leading to a detailed explanation of how to calculate and interpret these rates.
Insights
- Understanding chemical kinetics involves grasping fundamental concepts like concentration changes, moles, and partial pressure shifts as reactions progress, essential for further exploration in the subject.
- The calculation and interpretation of average rate of reaction, both mathematically and graphically, are crucial components in comprehending reaction rates and directionality of concentration changes.
- Instantaneous rate of reaction, stoichiometric considerations, and differential rate equations play significant roles in determining reaction rates, with the need for precision in calculating rates based on reactants and products.
Get key ideas from YouTube videos. It’s free
Recent questions
What is chemical kinetics?
The study of reaction rates and mechanisms.
Related videos

NCERT Wallah
CHEMICAL KINETICS in 55 Mins | Full Chapter Explanation + Most Important Topics Covered | Class 12

NCERT Wallah
Chemical Kinetics 01 | Rate of Reaction | Class 12th/CUET

Vora Classes NEET & Boards
Buniyaad NCERT Line by CHEMICAL KINETICS | Boards | NEET #neet #cbse #cbseboard #neet2024

Competition Wallah
CHEMICAL KINETICS in 70 minutes || Complete Chapter for NEET
The Organic Chemistry Tutor
Chemical Kinetics - Initial Rates Method
Summary
00:00
"Essential Concepts in Chemical Kinetics Explained"
- Chemical kinetics is a crucial chapter in class 12 chemistry, focusing on rates of reactions, factors affecting reaction rates, and reaction mechanisms.
- The chapter will be covered in three live sessions, with today being part one, followed by part two on Tuesday and part three on Friday.
- Basic concepts like the decrease in moles, concentration, and partial pressure of reactants as a reaction progresses, and the increase in moles, concentration, and partial pressure of products over time are essential to understand.
- Concentration is defined as the number of moles divided by the volume of the reaction vessel in liters, with reactant concentration decreasing and product concentration increasing as the reaction proceeds.
- The initial conditions at time T=0 show maximum moles of reactants and zero moles of products, with the moles of reactants decreasing and moles of products increasing over time.
- The decrease in moles of reactants leads to a decrease in the partial pressure of reactants, while the increase in moles of products results in an increase in the partial pressure of products.
- Graphs can be plotted to represent the changes in concentration, moles, and partial pressure of reactants and products over time, with reactant concentration decreasing and product concentration increasing as time progresses.
- The same trends apply to moles and partial pressure, with reactant moles, concentration, and partial pressure decreasing over time, and product moles, concentration, and partial pressure increasing.
- Understanding these basic concepts is crucial for grasping the fundamentals of chemical kinetics and will be essential for further discussions in the chapter.
- These foundational principles will set the stage for a comprehensive exploration of chemical kinetics, covering rates of reactions, factors influencing reaction rates, and the mechanisms involved in chemical reactions.
17:57
Analyzing Reaction Rates Through Graphs
- The text discusses six graphs related to a product, focusing on moles, concentration, and partial pressure versus time.
- It emphasizes that as a reaction starts, moles, concentration, and partial pressure of reactants decrease, while those of products increase.
- The first topic covered is the average rate of reaction, defined as the change in concentration of reactants or products in a given time interval.
- The formula for average rate of reaction is presented as the change in concentration divided by the time interval, denoted as r_a.
- An example is provided to illustrate the calculation of average rate of reaction with respect to reactants and products.
- The significance of the plus and minus signs in the formula is explained, indicating the direction of concentration changes.
- The graphical approach to understanding average rate of reaction is introduced, with graphs depicting concentration versus time for reactants and products.
- The calculation of average rate of reaction with respect to reactants and products in a specific time interval is demonstrated using the graph.
- The importance of adjusting the sign in the calculation to ensure a positive rate of reaction is highlighted.
- Overall, the text provides a detailed explanation of how to calculate and interpret average rate of reaction graphically and mathematically.
34:14
"Rate of Reaction Calculation and Understanding"
- Reactance calculation: Average rate of reaction calculated with respect to products and reactants, using minus sign for reactants and plus sign for products.
- Instruction to confirm understanding: Request for confirmation in the chat with thumbs up, emphasizing clarity on the concept.
- Telegram notes: Mention of handwritten notes to be shared on the Telegram channel "Wasim but chemistry official" after the session.
- Average rate of reaction: Introduction to the concept represented as "r a," followed by explanation of instantaneous rate of reaction as "r inss."
- Instantaneous rate of reaction definition: Defined as the change in concentration of reactant or product at a specific instant or a very small time interval.
- Comparison to physics concepts: Analogies drawn between average speed and instantaneous speed in physics to explain the difference between average and instantaneous rate of reaction.
- Geometric interpretation: Explanation of how instantaneous rate of reaction is represented as the slope of the tangent on a concentration versus time curve.
- Calculation of instantaneous rate: Detailed steps on calculating instantaneous rate of reaction with respect to products and reactants, considering the slope of the tangent.
- Rate of reaction as per stoichiometry: Introduction to the concept of rate of disappearance and rate of appearance for reactants and products, leading to the calculation of the overall rate of reaction.
- Calculation of rate of reaction: Formula provided for calculating the rate of reaction as the rate of disappearance or appearance of a reactant or product divided by the stoichiometric coefficient of the reactant or product.
52:35
Calculating Rate of Reaction in Chemistry
- Rate of reaction can be calculated with respect to reactants A, B, C, and D using the formula: 1/N change in concentration divided by time interval.
- Rate of reaction with respect to A starts with a minus sign, while rates with respect to B, C, and D start with a plus sign.
- The rate of reaction is constant within a specific time interval, regardless of the reactant being considered.
- The differential form of rate is used to calculate the rate of reaction, with units in moles per liter per second.
- For gaseous phase reactions at constant temperature, the rate of reaction can be expressed in terms of pressure as well as concentration.
- The differential form of rate in terms of pressure is calculated by substituting pressure values in place of concentration in the formula.
- The units of rate when calculated in terms of pressure are in ATM second inverse.
- To calculate the rate of reaction, determine whether it should be in moles per liter per second or ATM second inverse and use the appropriate formula.
- When given the rate of appearance of a product, the rate of disappearance of a reactant can be calculated using the differential form of rate.
- Equate the given rate of appearance with the rate of disappearance term to solve for the rate of disappearance of the specified reactant.
01:09:47
"Calculating Rates of Chemical Reactions"
- The formula for calculating the rate of disappearance of N2O5 is given as minus Delta N2O5 divided by Delta T, resulting in a rate of 1 mole per liter per second.
- A graph depicting concentration versus time for a reaction is provided, with the task to calculate the rate of reaction between 5 to 15 seconds, yielding a rate of 0.02 moles per liter per second.
- The differential form of rate for the given reaction is detailed, showcasing the rates in terms of H2O2, O2, and NH3.
- To calculate the rate of reaction for H2O2 changing from 0.5 mol to 0.05 mol in 15 seconds, the formula is applied, resulting in a rate of -0.4 divided by 10, equaling 0.02 moles per liter per second.
- The rate of appearance of O2 is to be determined, with the differential form of rate used to equate the change in H2O2 to the change in O2, leading to a rate of appearance of 0.02 moles per liter per second.
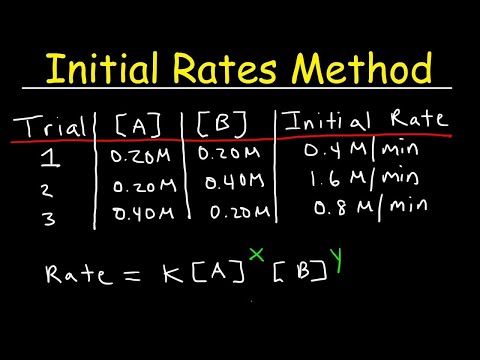
- A question is presented to find the values of X and Y in a given equation, utilizing logarithmic calculations and differential rate equations to determine X as 2 and Y as 1.
- Another question tasks the determination of the relationship between rate constants K1, K2, and K3 for a reaction, resulting in the relation K1 = K2 / 2 = 2 * K3.
- Homework is assigned involving calculating the rate of reaction in moles per liter per second and in ATM per second for a given reaction, with answers to be provided in the comments section.




