How to Find Number of Neutrons (Two methods, both easy)
chemistNATE・4 minutes read
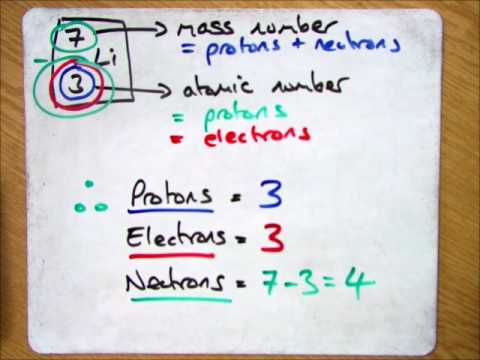
To determine the number of neutrons in a particle, subtract the atomic number from the mass number, which is crucial for understanding isotopes. If the mass number is not provided, round the atomic mass and subtract the atomic number to find the neutron count.
Insights
- The number of neutrons in a particle can be calculated by subtracting the atomic number (protons) from the mass number of the particle, which is crucial in understanding the composition of atoms.
- Isotopes of the same element have different mass numbers, impacting the number of neutrons in a particle; knowing this distinction is essential for accurate neutron count calculations.
Get key ideas from YouTube videos. It’s free
Recent questions
How do you find the number of neutrons in a particle?
By subtracting the atomic number from the mass number.
What should you do if the mass number is not provided?
Round the atomic mass and subtract the atomic number.
Why is understanding isotopes important in determining neutron count?
Isotopes have varying mass numbers affecting neutron count.
How do you calculate neutron count for isotopes?
Subtract the atomic number from the mass number.
How can you differentiate between isotopes of an element?
By comparing their mass numbers.
Related videos
Mr Barnes
Calculating the Protons, Neutrons and Electrons for an Atom

Khan Academy
Worked example: using the mass number equation | High school chemistry | Khan Academy

Wayne Breslyn
How to Find the Mass Number of an Element

Cognito
GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2

Allied Schools
Class 10 - Physics - Chapter 18 - Lecture 1 - 18.1 Atom and Atomic Nucleus - Allied Schools
Summary
00:00
Calculating Neutrons in Atoms: Essential Guide
- To find the number of neutrons in a particle, you need to subtract the atomic number (protons) from the mass number of the particle. The mass number is given by the symbol with a number in the upper left corner or after a hyphen, while the atomic number is a constant value for all atoms of the same element.
- If your teacher doesn't provide the mass number of the particle, round the atomic mass from the periodic table to the nearest whole number and then subtract the atomic number to determine the number of neutrons. This method applies when specific isotopes are not indicated.
- Understanding the difference between isotopes is crucial in determining the number of neutrons in a particle. Isotopes of the same element have varying mass numbers, leading to different neutron counts, which can be calculated by subtracting the atomic number from the mass number.




