GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
Cognito・5 minutes read
Atoms consist of protons, neutrons, and electrons, with the number of protons determining the element; the periodic table organizes elements by atomic number, with isotopes having varying numbers of neutrons but the same number of protons. The relative atomic mass of an element is calculated by considering the abundance of each isotope, providing the average mass of an atom.
Insights
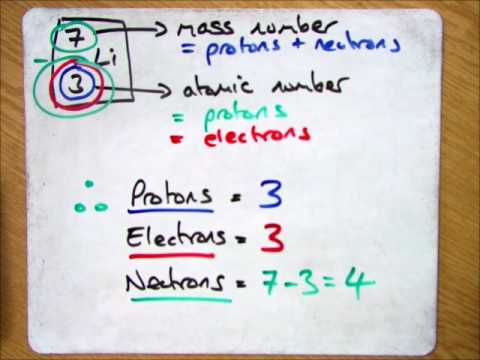
- The number of protons in an atom determines the element it belongs to, with hydrogen being the simplest element having one proton, followed by helium with two protons, two neutrons, and two electrons.
- Isotopes are versions of elements with different neutron numbers but the same proton count; for example, carbon-12 and carbon-13 both have six protons but varying neutron counts, showcasing the diversity within elements.
Get key ideas from YouTube videos. It’s free
Recent questions
What are the components of an atom?
An atom consists of a central nucleus containing protons and neutrons, with electrons orbiting around it.
How is the periodic table organized?
The periodic table organizes elements based on their atomic number, which represents the number of protons in the nucleus of an atom.
What are isotopes?
Isotopes are different forms of the same element that have the same number of protons but varying numbers of neutrons.
How is the relative atomic mass calculated?
The relative atomic mass of an element is determined by summing the abundance of each isotope multiplied by its mass and dividing by the total abundance.
Can you provide an example of an isotope?
An example of an isotope is carbon-12, which has six protons and six neutrons, while carbon-13 has six protons and seven neutrons.
Related videos
Summary
00:00
"Atomic Structure, Periodic Table, Isotopes, Mass"
- An atom consists of a central nucleus with protons, neutrons, and orbiting electrons; the number of protons determines the element, with hydrogen being the smallest having one proton and one electron, followed by helium with two protons, two neutrons, and two electrons.
- The periodic table organizes around 100 elements, each represented by a nuclear symbol with the atomic number (proton count) unique to that element; for example, helium has an atomic number of two, while carbon has six protons.
- Isotopes are different forms of the same element with varying numbers of neutrons but the same number of protons; for instance, carbon-12 has six protons and neutrons, while carbon-13 has six protons and seven neutrons.
- The relative atomic mass of an element is calculated by summing the abundance of each isotope multiplied by its mass, then dividing by the total abundance; for copper, the relative atomic mass is 63.6, representing the average mass of a copper atom.