Biomolecules in 100 Minutes | Chemistry Chapter 10 | Full Chapter Revision Class 12th
NCERT Wallah・2 minutes read
Glucose and fructose are key components in various biochemical processes, while carbohydrates are classified into different groups based on their structure and function. Additionally, proteins, amino acids, enzymes, vitamins, nucleic acids, and hormones play essential roles in maintaining the body's health and functionality.
Insights
- Glucose and fructose, despite sharing the same molecular formula, have different functional groups, with glucose being a reducing sugar and fructose being a keto hexose.
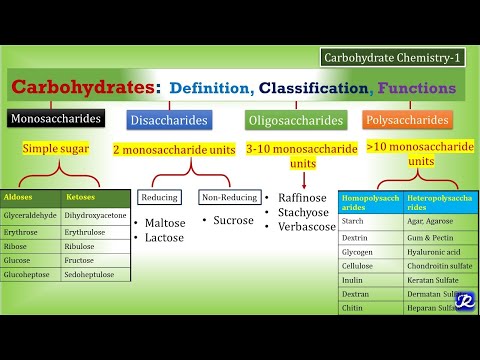
- Carbohydrates are classified into monosaccharides, oligosaccharides, and polysaccharides, each with distinct structures and functions, while enzymes act as biological catalysts with specific functions and optimal conditions for biochemical reactions.
Get key ideas from YouTube videos. It’s free
Recent questions
What are carbohydrates?
Carbohydrates are polyhydroxy aldehydes or ketones.
Related videos
N'JOY Biochemistry
1:Carbohydrates-Definition, Classification, Functions | Carbohydrate Chemistry 1| Biochemistry

Anatomy & Physiology with Dr. J
PHYL 142 | Digestive | Biological Macromolecules

Amoeba Sisters
Biomolecules (Updated 2023)

Reggie Cobb
Ch 03 Lecture Presentation Video

Nursing Wallah by PW
Carbohydrates | Fats | Nutrition | BSc Nursing 1st Year | Lifeline Batch
Summary
00:00
"Glucose and Fructose: Carbohydrates and Sugars"
- Glucose and fructose combine to form C6H12O6, known as glucose and fructose.
- Glucose is a reducing sugar that can reduce the solution with a tolerance reagent.
- Carbohydrates are defined as optically active polyhydroxy aldehydes or ketones.
- Carbohydrates are classified into monosaccharides, oligosaccharides, and polysaccharides.
- Sugars are crystalline, water-soluble, and sweet, collectively known as sugars.
- Glucose is found in fruits, honey, and ripe grapes, with a molecular formula of C6H12O6.
- Hydrolyzing sucrose yields glucose and fructose, both with the same molecular formula but different functional groups.
- Starch can be hydrolyzed into glucose by adding water, dilute acid, and heat.
- Glucose reacts with hydroxylamine to form an oxime, revealing the presence of a carbonyl group.
- Glucose reacts with bromine to determine the presence of a ketone or aldehyde group.
21:10
"Glucose: Stable Compound with Unique Structure"
- Carbon is unstable due to Gem Dal, with two O's on one carbon.
- Glucose is a stable compound with a different Hydroxy group on the same carbon.
- Glucose has six carbon atoms in a straight chain.
- Glucose has a primary alcohol functional group.
- Glucose has a carbonyl functional group near the Aldehyde functional group.
- Glucose has five Hydroxy groups on different carbons.
- Glucose has a primary alcohol functional group near the secondary ones.
- Glucose is Dexo Rotatory due to its structure.
- Glucose exists in open and closed chain structures.
- Fructose is a keto hexose with a different structure from glucose.
39:19
"Understanding Sugars and Polysaccharides in Biochemistry"
- Sucrose is an example of non-reducing sugar, leading to questions about why it's called invert sugar.
- Maltose consists of a glycosidic linkage of alpha D glucose and another alpha D glucose at C2 and C4.
- Sucrose hydrolase breaks down sucrose into glucose and fructose, while maltose hydrolase yields two units of glucose.
- Lactose contains a glycosidic linkage of beta D galactose and beta D glucose at C1 and C4.
- Maltose and lactose are both reduced sugars, with specific structures involving alpha D glucose and beta D galactose.
- Starch, cellulose, and glycogen are polysaccharides with distinct structures and functions.
- Starch is a polymer of alpha glucose with components of amylose and amylopectin.
- Amylose is a water-soluble component of starch with a straight chain structure and glycosidic linkages at C1 and C4.
- Amylopectin is insoluble in water, branching from alpha D glucose units at C1 and C6 with glycosidic linkages at C1 and C4.
- Cellulose is a straight chain polymer of beta D glucose units with glycosidic linkages at C1 and C4, forming the cell wall in plants.
56:56
Understanding Amino Acids and Carbosynths
- Amino acids consist of an amine group and a carbocysteine off the amino group.
- CarboSynth Neutral contains glison, wally, El Nino, and Jasin.
- Amino and Carbosynths are basic in nature, with amino groups determining the acidity.
- A higher amino group number results in a basic amino acid.
- Two amino groups on the carboxylic acid make an amino acid basic.
- Amino group dominance leads to basic amino acids, while carbocysteine dominance results in acidic amino acids.
- Non-essential amino acids can be synthesized by the body, while essential ones must be obtained from the diet.
- Essential amino acids are crucial for the body and cannot be synthesized internally.
- Amino acids are optically active, except for glisson, which is optically inactive.
- Amino acids form proteins through peptide linkages, with the number of linkages equal to the number of amino acid residues minus one.
01:18:17
Protein Folding, Denaturation, Enzymes, Vitamins, DNA vs RNA
- Poly peptide chain stretched by stretching it, giving second and third stretches.
- Stretching Poly Peptide Chains to maximum extension and arranging them side by side with intermolecular hydrogen bonding.
- Formation of beta plated sheets in the secondary structure of poly peptide chains.
- Overall folding of poly peptide chains starting with the secondary structure.
- Denaturation of proteins due to physical changes like temperature or pH alterations, leading to unfolding and loss of biological activity.
- Common examples of denaturation like egg white solidifying or milk curdling.
- Enzymes as biological catalysts catalyzing biochemical reactions, with specific functions and optimum working conditions.
- Classification of vitamins into fat-soluble and water-soluble forms, essential for healthy growth and disease prevention.
- Deficiencies and effects of various vitamins like Vitamin C deficiency causing scurvy or Vitamin D deficiency leading to rickets.
- Differences between DNA and RNA in terms of sugar units, bases, and nucleotide structures, forming nucleic acids with specific linkages.
01:35:40
Genetic material, hormones, and their functions
- Nucleic acids consist of two strands that form hydrogen bonds between specific pairs, such as CG and AT, creating a double bond.
- RNA is single-stranded, while DNA is double-stranded, with RNA sometimes folding back on itself.
- Hormones are chemical substances produced by endocrine glands, released into the bloodstream to regulate body functions, with different types classified based on their chemical nature and functions.




