Orbitals
Saylor Academy・14 minutes read
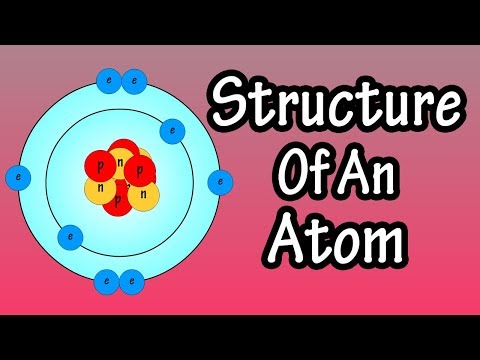
The structure of atoms involves a nucleus with electrons forming a smear around it, not in fixed orbits but as a probability function. Different elements have unique electron configurations based on energy shells, with electrons following specific rules for filling orbitals.
Insights
- Electrons in an atom are not in fixed orbits but exist as a probability function around the nucleus, described by the Heisenberg uncertainty principle. This challenges the traditional view of electrons moving in predictable paths like planets around a star.
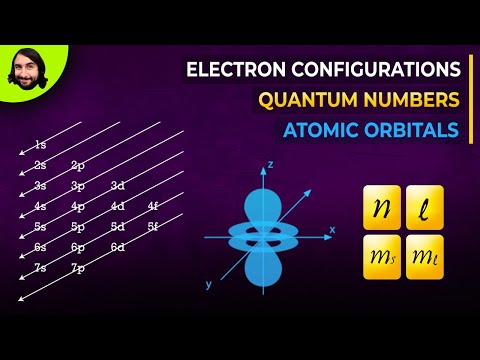
- The electron configuration of atoms follows a specific pattern based on energy shells denoted by the quantum number 'n', with each shell accommodating a certain number of electrons. This structured filling of orbitals determines an element's chemical properties and behavior.
Get key ideas from YouTube videos. It’s free
Recent questions
What is the Heisenberg uncertainty principle?
It states that the position and momentum of a particle cannot be precisely determined simultaneously.
How are electrons described in an atom?
As a smear around the nucleus due to the Heisenberg uncertainty principle.
What is the Bohr model?
A model conceptualizing electrons as planets around a star.
How do electrons move between energy states?
By absorbing or emitting energy in the form of light waves.
What is the electron configuration of lithium?
1s2 2s1
Related videos

Bryson Chemistry
Electron Shells, Sub-shells and Orbitals

PassChem: Sponholtz Productions
Introduction to the Atom (English)
Professor Dave Explains
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Whats Up Dude
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons

The Chemistry Tutor
Atomic Structure (full topic) | A Level
Summary
00:00
Atomic Structure and Electron Configurations
- The nucleus at the center of an atom is a small fraction of the total volume, with electrons described as a smear around it due to the Heisenberg uncertainty principle.
- Electrons in an atom are not in orbits like planets around the Sun but can be described as a probability function around the nucleus.
- The 1s orbital is a sphere around the nucleus with no strict boundary, denser towards the center, indicating a higher probability of finding the electron there.
- The Bohr model, named after Niels Bohr, conceptualizes electrons as planets revolving around a star, useful for understanding energy states.
- Energy levels in atoms can change, affecting the electron's path, similar to a planet's orbit becoming more elliptical with more energy.
- Light waves can give energy to electrons, moving them to higher energy states, emitting photons when returning to lower states.
- Electrons fill different orbitals based on energy shells denoted by the number 'n', corresponding to periods in the periodic table.
- Hydrogen's electron configuration is 1s1, with one electron in the 1s orbital, shaped like a sphere.
- Helium's electron configuration is 1s2, with two electrons in the 1s orbital, following the rule of two electrons per subshell.
- Lithium's electron configuration progresses to 1s2 2s1, filling the 1s orbital with two electrons and moving the third electron to the 2s orbital in the second energy shell.




