Nuclear Chemistry & Radioactive Decay Practice Problems
The Organic Chemistry Tutor・17 minutes read
Mercury 201 has 80 protons, 121 neutrons, and 80 electrons, making it an alpha particle with a mass of 4 and a charge of 2. Iodine 131 undergoing beta decay converts into Xenon (Xe) with an atomic number of 54, changing the neutron-proton ratio to increase stability.
Insights
- Beta decay is a crucial process that converts a neutron into a proton, exemplified by Iodine 131 transforming into Xenon, thus impacting the stability of isotopes like Carbon 14 by altering their neutron-proton ratio.
- Nuclear fission, as observed in Uranium 235, splits heavy elements into lighter ones, generating three neutrons, while nuclear fusion combines lighter elements like hydrogen to create heavier elements, a fundamental mechanism like the sun's energy production.
Get key ideas from YouTube videos. It’s free
Recent questions
What is the process of alpha decay?
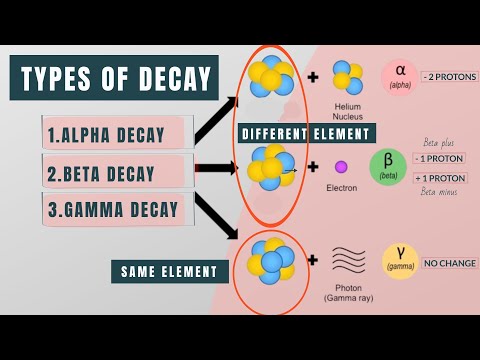
Alpha decay involves the emission of an alpha particle, which is the nucleus of a helium atom consisting of 2 protons and 2 neutrons.
Related videos

FuseSchool - Global Education
Stable and Unstable Nuclei | Radioactivity | Physics | FuseSchool

Allied Schools
Class 10 - Physics - Chapter 18 - Lecture 1 - 18.1 Atom and Atomic Nucleus - Allied Schools

Whats Up Dude
Atomic Structure And Electrons - Structure Of An Atom - What Are Atoms - Neutrons Protons Electrons

Cognito
GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
Dr. Paulien Moyaert
Different types of decay | Alpha vs. Beta vs. Gamma decay | Visual Explanation
Summary
00:00
Decay processes in nuclear chemistry explained
- Mercury 201 has 80 protons, 121 neutrons, and 80 electrons.
- Nucleons in Mercury 201 total 201, with 80 protons and 121 neutrons.
- An alpha particle is the nucleus of a helium atom, with a mass of 4 and a charge of 2.
- Thorium 230 undergoing alpha decay forms Radium (Ra) due to atomic number changes.
- Iodine 131 undergoing beta decay produces Xenon (Xe) with an atomic number of 54.
- Beta decay converts a neutron into a proton, as seen in the transformation of Iodine 131 to Xenon.
- Radium 227 is the unknown element produced when Uranium 238 is struck by a neutron.
- Carbon 14, with a neutron-proton ratio of 1.33, is likely to undergo radioactive decay.
- Carbon 14 would use beta decay to increase nuclear stability by decreasing its neutron-proton ratio.
- Alpha decay, positron decay, gamma decay, and electron capture are not suitable for stabilizing Carbon 14's neutron-proton ratio.
20:31
Radioactive Decay and Nuclear Reactions Explained
- Carbon 14 has a neutron-proton ratio of 1.33, while nitrogen has a ratio of 1, making beta decay ideal for carbon 14 to increase stability by reducing the ratio to 1.
- Elements with an atomic number of 84 or more, like radon, uranium, thorium, and radium, undergo radioactive decay, except for lead 206, which remains stable.
- Nuclear fission involves breaking a heavy element like uranium 235 into lighter elements, producing three neutrons, while nuclear fusion combines lighter elements like hydrogen to form heavier elements, as seen in the sun's energy generation.




