What is the Periodic Table? How are Elements Organized?
Math and Science・2 minutes read
The periodic table organizes elements by atomic number and properties to predict element behavior, discover new elements, and understand reactions, aiding in various scientific fields. Elements are grouped into periods and groups based on electron configuration, with noble gases being unreactive due to their stable electron configuration, while metals, non-metals, and metalloids exhibit distinct properties and are essential for various applications.
Insights
- The periodic table categorizes elements based on atomic number, electron configuration, and chemical traits, aiding in predicting element behavior and fostering discoveries in various scientific fields.
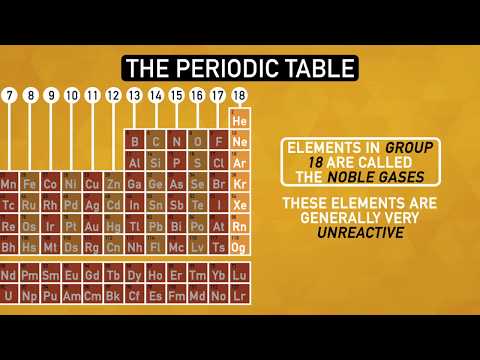
- Elements within the same group share similar properties due to their valence electron configuration, with noble gases on the far right being unreactive, while metals, non-metals, and metalloids occupy distinct sections, each crucial in understanding chemical reactions and material design.
Get key ideas from YouTube videos. It’s free
Recent questions
How does the periodic table organize elements?
By atomic number, electron configuration, and properties.
What are noble gases and why are they unreactive?
Elements on the far right column with full outer shells.
How do elements in the same group behave?
Share similar chemical and physical properties.
What are metalloids and where are they located on the periodic table?
Elements with properties between metals and non-metals.
Why are transition metals located in the middle of the periodic table?
Transition metals have varying electron configurations.
Related videos

Lincoln Learning Solutions
The Periodic Table Explained

Sir Tarun Rupani
Periodic Table, Periodic Properties and Variations of Properties Class 10 ICSE | @sirtarunrupani
American Chemical Society
How The Periodic Table Organizes the Elements | Chemistry Basics

Professor Dave Explains
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity

The Organic Chemistry Tutor
Intro to Chemistry, Basic Concepts - Periodic Table, Elements, Metric System & Unit Conversion
Summary
00:00
"The Periodic Table: Element Organization and Behavior"
- The periodic table organizes elements by atomic number, electron configuration, and chemical properties.
- It enables scientists to predict element behavior, discover new elements, and understand chemical reaction patterns.
- The table comprises just over a hundred elements, forming the basis of all substances encountered in daily life.
- Elements are grouped into periods (rows) based on electron shells and groups (columns) based on valence electrons.
- Elements within the same group share similar chemical and physical properties due to their valence electron configuration.
- The noble gases, located in the far right column, are unreactive due to their full outer shell.
- The periodic table aids in predicting element reactions based on their column placement.
- It is utilized in various scientific fields like chemistry, physics, and material science for understanding reactions and designing materials.
- Combining elements can create compounds with vastly different properties from their parent elements.
- The table is arranged by increasing atomic number, with each element having a unique number of protons and neutrons in its nucleus.
14:31
Chemistry: Periodic Table and Elemental Properties
- Chemistry involves chemical reactions that occur in a periodic manner.
- The periodic table organizes elements in a specific order based on their properties.
- Noble gases are unreactive elements located on the right side of the table.
- Reactive metals are found just after the noble gases in the table.
- The layout of the periodic table is influenced by electron configurations around atoms.
- Elements in the same group or column exhibit similar properties.
- The left side of the table consists of metals, while the right side has non-metals.
- Metalloids are elements located on the border between metals and non-metals.
- Silicon, a metalloid, is crucial for making computer chips due to its unique properties.
- Carbon, located above silicon, is essential as the building block of life on Earth.
27:44
"Periodic Table: Carbon, Metals, Noble Gases"
- Elements on the periodic table require carbon to exist.
- The table is divided into metals on the left, non-metals on the right, and transition metals in the middle.
- An additional row below the periodic table contains high atomic number atoms.
- Noble gases are non-reactive due to stable electrons.
- Elements aim to mimic noble gas electron configurations by gaining or losing electrons.
- Elements in columns indicate the number of electrons they want to gain or lose.
- Elements on the left side of the table tend to lose electrons easily.
- Elements on the right side of the table tend to gain electrons readily.
- Middle elements can also give up electrons but are less predictable.
- The periodic table structure is influenced by electron configurations and spacing.
40:57
"Periodic Table Groups and Element Classification"
- The periodic table has had different labels for groups in the past, with some literature still using old labels like 1A and 1B.
- The current standard labels groups as 1A, 2A, 3A, 4A, 5A, 6A, 7A, and 8A, but variations may exist in different sources.
- Group 1A is known as alkali metals, Group 2A as alkaline Earth metals, Group 7A as halogens, and Group 8A as noble gases.
- Metals are on the left side of the table, non-metals on the right, and metalloids near the stair-step line.
- Metals are shiny, ductile, good conductors of electricity and heat, while non-metals are brittle and non-conductive.
- Main group elements, labeled 1A to 8A, are crucial in basic chemistry and organic chemistry studies.
- Hydrogen, although not a metal, is placed with metals due to its unique electron behavior, being able to lose or gain electrons.
- Elements like chromium are classified as metals if they are on the left side of the periodic table.
- Non-metals like helium are found on the right side of the table and are non-reactive.
- Metalloids like arsenic are located near the boundary between metals and non-metals, exhibiting properties of both.
54:51
"Periodic Table: Electron Distribution and Reactivity"
- The periodic table's unique shape is due to electron distribution around atoms, impacting its appearance.
- Increasing protons in atoms lead to periodic patterns, with noble gases being stable due to their electron configuration.
- Elements near noble gases are reactive, aiming to mimic their stability by gaining or losing electrons.
- The periodic table consists of periods and groups, with specific names for different groups like alkali metals, alkaline earth metals, halogens, and noble gases.




