Types of decay | Nuclear chemistry | Chemistry | Khan Academy
Khan Academy・13 minutes read
The text discusses the various types of radioactive decay, including alpha, beta, positron emission, and gamma decay, explaining how atomic structure changes during these processes. It provides specific examples, such as uranium-238 transforming into thorium-234 through alpha decay and iodine undergoing beta decay, while emphasizing the preservation of mass and the stabilization of resulting isotopes.
Insights
- The discussion reveals that the stability of atomic nuclei is influenced by various decay processes, including alpha and beta decay, where unstable nuclei emit particles like alpha particles (helium nuclei) or electrons, leading to transformations of elements and changes in their atomic structure. For instance, during alpha decay, uranium-238 loses 4 in atomic mass and 2 in atomic number to become thorium-234, demonstrating the intricate relationship between nuclear stability and decay mechanisms.
- Additionally, the text highlights the significance of positron emission and gamma decay as alternative decay processes, where positron emission involves a proton converting to a neutron and emitting a positron, while gamma decay releases energy without altering the number of protons or neutrons. The example of 7-beryllium decaying into 7-lithium illustrates how positron emission maintains mass while changing atomic number, emphasizing the diverse pathways through which atomic nuclei can achieve stability.
Get key ideas from YouTube videos. It’s free
Recent questions
What is alpha decay in simple terms?
Alpha decay is a process where an unstable atomic nucleus emits an alpha particle, which consists of two protons and two neutrons. This emission results in a decrease of the atomic mass number by four and the atomic number by two, effectively transforming the original element into a new one. For instance, when uranium-238 undergoes alpha decay, it releases an alpha particle and becomes thorium-234. This process not only changes the identity of the element but also illustrates the fundamental nature of nuclear stability and the mechanisms that lead to the transformation of elements in the periodic table.
How does beta decay occur?
Beta decay occurs when a neutron in an atomic nucleus transforms into a proton, resulting in the emission of an electron, known as a beta particle. This transformation increases the atomic number by one while keeping the mass number unchanged, thus creating a new element. For example, iodine, which has 53 protons, can undergo beta decay to become an isotope with 54 protons by converting one of its neutrons into a proton. The emitted beta particle carries away the negative charge, allowing the new isotope to stabilize by acquiring electrons from its surroundings, ultimately returning to a neutral state.
What is gamma decay?
Gamma decay is a nuclear process where an unstable nucleus releases energy in the form of gamma rays without changing the number of protons or neutrons within the nucleus. This release of high-energy radiation occurs as particles within the nucleus reconfigure themselves to reach a more stable state. Unlike alpha and beta decay, gamma decay does not result in the transformation of one element into another; instead, it is a way for the nucleus to shed excess energy. Gamma rays are highly penetrating and can pose significant hazards to living organisms, making understanding this decay process crucial in fields such as nuclear physics and radiation safety.
What happens during positron emission?
During positron emission, a proton in the nucleus of an atom is converted into a neutron, resulting in the emission of a positron, which is a particle with the same mass as an electron but with a positive charge. This process decreases the atomic number by one while keeping the mass number unchanged, thus forming a new element. For example, when beryllium-7 undergoes positron emission, it transforms into lithium-7. The emitted positron quickly interacts with electrons in the environment, leading to annihilation events that produce gamma radiation, highlighting the intricate balance of particle interactions in atomic decay processes.
How does alpha decay affect atomic structure?
Alpha decay significantly alters the atomic structure of an element by reducing both its atomic mass and atomic number. When an unstable nucleus emits an alpha particle, which is essentially a helium nucleus composed of two protons and two neutrons, the original element loses four units of mass and two units of atomic number. This transformation results in the formation of a new element. For instance, uranium-238, upon undergoing alpha decay, becomes thorium-234. The loss of protons and neutrons not only changes the identity of the element but also impacts its chemical properties and stability, illustrating the dynamic nature of atomic interactions and decay processes in nuclear chemistry.
Related videos
Dr. Paulien Moyaert
Different types of decay | Alpha vs. Beta vs. Gamma decay | Visual Explanation
The Organic Chemistry Tutor
Half Life Chemistry Problems - Nuclear Radioactive Decay Calculations Practice Examples

FuseSchool - Global Education
Stable and Unstable Nuclei | Radioactivity | Physics | FuseSchool

Pla Academy: IGCSE and A level buddy
O Level Physics 5054 Unit 5 Nuclear Physics #o_level_physics

Tyler DeWitt
Nuclear Half Life: Intro and Explanation
Summary
00:00
Nuclear Decay Processes Explained Simply
- The discussion centers on the stability of electrons in atoms, highlighting that the nucleus also experiences interactions and potential instability that can lead to decay processes, although the mechanics are complex and beyond first-year chemistry scope.
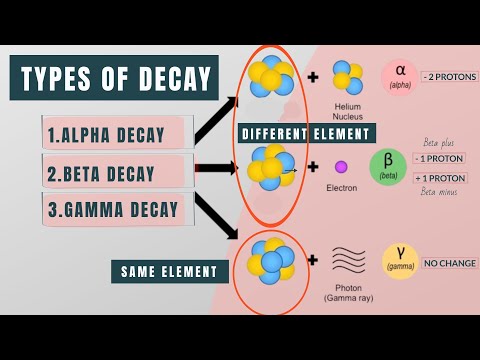
- Alpha decay occurs when an unstable nucleus emits an alpha particle, which consists of two protons and two neutrons, resulting in a decrease of the atomic mass number by four and the atomic number by two, transforming the original element into a new one.
- For example, if an element has 'p' protons and undergoes alpha decay, it will have 'p - 2' protons and 'n - 2' neutrons, where 'n' is the original number of neutrons, leading to a new element with an atomic mass of 'p + n - 4'.
- The emitted alpha particle is identified as a helium nucleus, which has a mass of four and a +2 charge due to the two protons, effectively making it a helium ion.
- Beta decay occurs when a neutron in the nucleus transforms into a proton by emitting an electron, resulting in an increase of the atomic number by one while the mass number remains unchanged, thus creating a new element.
- In beta decay, if an element has 'p' protons and 'n' neutrons, after decay it will have 'p + 1' protons and 'n - 1' neutrons, maintaining the same mass number of 'p + n'.
- Positron emission is another decay process where a proton converts into a neutron by emitting a positron, which has the same mass as an electron but a positive charge, resulting in a decrease of the atomic number by one while the mass number remains unchanged.
- For positron emission, starting with 'p' protons and 'n' neutrons, the new configuration will have 'p - 1' protons and 'n + 1' neutrons, again keeping the mass number as 'p + n'.
- Gamma decay involves the release of energy without changing the number of protons or neutrons in the nucleus, as particles reconfigure themselves, emitting high-energy gamma rays, which are hazardous.
- An example problem illustrates positron emission, where 7-beryllium (4 protons) decays into 7-lithium (3 protons), confirming that the mass remains constant while the atomic number decreases, indicating the emission of a positron.
13:12
Nuclear Decay Processes and Element Transformations
- Uranium-238 undergoes alpha decay, transforming into thorium-234 by releasing an alpha particle, which is a helium nucleus with an atomic mass of 4 and an atomic number of 2. This process results in a decrease of 4 in atomic mass and 2 in atomic number, while thorium quickly loses two electrons to maintain a neutral charge, and the released helium nucleus rapidly acquires electrons from its surroundings to stabilize.
- In beta decay, iodine with 53 protons transforms into an isotope with 54 protons by converting a neutron into a proton and releasing an electron, known as a beta particle. Although the increase in protons suggests a positive charge, the new isotope quickly stabilizes by acquiring electrons from its environment, thus returning to a neutral state.
- Radon-222 decays into polonium-218, with a decrease of 4 in atomic mass and 2 in atomic number, also releasing a helium nucleus, indicating alpha decay. The element polonium was named in honor of Poland, reflecting the historical context of its discovery around the late 1800s when Poland was not an independent nation.




