QUANTITATIVE CHEMISTRY - GCSE Chemistry (AQA Topic C3)
Science Shorts・2 minutes read
The conservation of mass in chemical reactions requires balancing equations and calculating moles using the formula moles = mass (g) / relative atomic mass (RAM), exemplified by methane producing water. Additionally, metrics like percentage yield and atom economy are crucial for assessing chemical efficiency, demonstrated through calculations resulting in a 50% yield and 55% atom economy in a reaction involving methane.
Insights
- The principle of conservation of mass in chemical reactions requires that the mass of reactants equals the mass of products, emphasizing the importance of balancing chemical equations; for instance, carbon dioxide (CO2) has a relative formula mass of 44 g/mol, calculated from its constituent elements.
- In chemical processes, metrics like percentage yield and atom economy help evaluate efficiency; for example, if 20 g of reactants produce 10 g of ammonia, the percentage yield is 50%, while the atom economy for the methane reaction is 55%, highlighting how effectively reactants are converted into desired products.
Get key ideas from YouTube videos. It’s free
Recent questions
What is the conservation of mass?
The conservation of mass is a fundamental principle in chemistry stating that mass cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants must equal the total mass of the products. When balancing chemical equations, it is essential to ensure that the number of atoms of each element is the same on both sides of the equation. This principle is crucial for accurately predicting the outcomes of chemical reactions and is foundational for stoichiometry, which involves the calculation of reactants and products in chemical processes.
How do you calculate moles?
To calculate the number of moles in a substance, you can use the formula: moles = mass (in grams) / relative atomic mass (RAM). This formula allows you to convert the mass of a substance into moles, which is a standard unit in chemistry for quantifying the amount of a substance. For example, if you have 64 grams of methane (CH4) and know that its RAM is 16, you would divide 64 by 16 to find that you have 4 moles of methane. This calculation is essential for understanding the relationships between different substances in a chemical reaction.
What is percentage yield in chemistry?
Percentage yield is a measure of the efficiency of a chemical reaction, calculated by comparing the actual yield of a product to the theoretical yield. It is expressed as a percentage and provides insight into how much of the expected product was actually produced. For instance, if a reaction is expected to produce 20 grams of a product but only yields 10 grams, the percentage yield would be calculated as (10 g / 20 g) × 100, resulting in a 50% yield. This metric is important for evaluating the effectiveness of reactions and can help chemists optimize their processes.
What is atom economy?
Atom economy is a concept in green chemistry that measures the efficiency of a chemical reaction in terms of how well the reactants are converted into useful products. It is calculated using the formula: (RAM of desired product / total RAM of reactants) × 100. A higher atom economy indicates a more efficient reaction, as it means that a greater proportion of the reactants are being transformed into the desired product rather than by-products. For example, in a reaction producing carbon dioxide and water from methane, if the RAM of the desired product is considered, the atom economy can be calculated to assess the sustainability of the reaction.
How do you calculate mass from moles?
To calculate mass from moles, you can rearrange the formula used for determining moles. The formula is mass = moles × relative atomic mass (RAM). This means that if you know the number of moles of a substance and its RAM, you can easily find the mass. For example, if you have 8 moles of water (H2O), and the RAM of water is 18 g/mol, you would multiply 8 by 18 to find that the mass of water produced is 144 grams. This calculation is crucial for quantifying substances in chemical reactions and ensuring that the correct amounts are used in experiments.
Related videos

The Organic Chemistry Tutor
Solution Stoichiometry - Finding Molarity, Mass & Volume

The Organic Chemistry Tutor
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
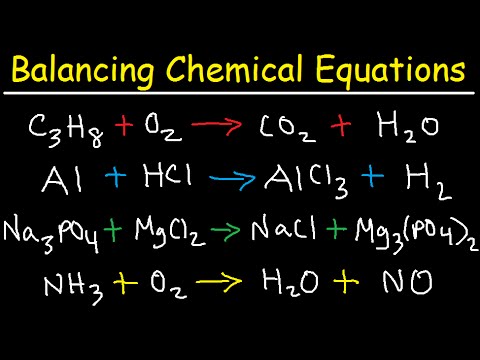
The Organic Chemistry Tutor
Introduction to Balancing Chemical Equations

Professor Dave Explains
The Mole: Avogadro's Number and Stoichiometry

Exam Winner Plus One
+1 Chemistry Onam Exam | Chapter 1 | Some Basic Concepts Of Chemistry | Exam Winner +1
Summary
00:00
Balancing Chemical Reactions and Yield Calculations
- The conservation of mass in chemical reactions necessitates balancing equations, where the total mass of reactants equals the total mass of products; for example, the relative formula mass of carbon dioxide (CO2) is calculated as 12 (carbon) + 2 × 16 (oxygen) = 44 g/mol.
- To determine the number of moles, use the formula: moles = mass (g) / relative atomic mass (RAM); for instance, 64 g of methane (CH4) with a RAM of 16 results in 4 moles of methane, and since the stoichiometry of the reaction indicates 2 moles of water (H2O) are produced for every mole of methane, this yields 8 moles of water.
- The mass of water produced can be calculated by rearranging the formula to mass = moles × RAM; thus, 8 moles of water (with a RAM of 18) results in 144 g of water, and this calculation can be adapted for other units like kilograms or tons, as long as consistent units are used throughout.
- Percentage yield and atom economy are important metrics in chemical reactions; for example, if 20 g of reactants yield 10 g of ammonia, the percentage yield is 50%. Atom economy is calculated as (RAM of desired product / total RAM of reactants) × 100; in the methane reaction, this results in an atom economy of 55% when considering the RAM of CO2 and water.




