How to Balance Chemical Equations
MooMooMath and Science・2 minutes read
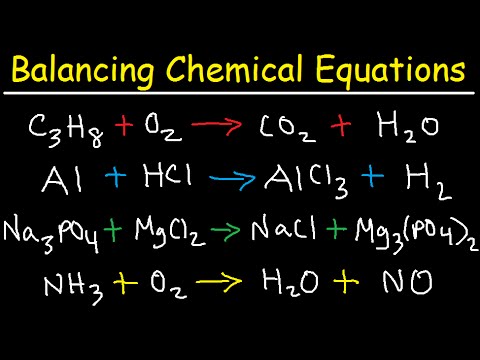
Balancing chemical equations involves counting atoms on each side, starting with metals, then nonmetals, and adjusting coefficients to ensure balance. It is crucial to identify the number of each element on both sides, starting with metals and adjusting coefficients before moving on to nonmetals like oxygen, and multiplying the smallest common factor for accurate balance.
Insights
- When balancing chemical equations, it is crucial to start with metals, then nonmetals, and finally hydrogen or other elements, adjusting coefficients to ensure an equal number of each element on both sides.
- The key to accurately balancing equations involving multiple elements like potassium, chlorine, and oxygen lies in meticulous counting and coefficient adjustment for each element individually, maintaining equilibrium on both sides for a balanced equation.
Get key ideas from YouTube videos. It’s free
Recent questions
How do you balance chemical equations?
By counting atoms on each side and adjusting coefficients.
What is the first step in balancing equations?
Drawing a line down the middle.
Which elements should be balanced first?
Metals, then nonmetals.
How do you adjust coefficients in equations?
To balance the equation accurately.
Why is it important to balance chemical equations?
To maintain the law of conservation of mass.
Related videos
The Organic Chemistry Tutor
Introduction to Balancing Chemical Equations

JEE Nexus by Unacademy
Redox Reactions Class 11 | JEE Main & Advanced

Iraj Parajuli Classes-For SEE and 11,12
NEB-11 Chemistry||Lecture-1||Oxidation no. method to balance rxn||Full concept¬e||watch in 1080p

Science Shorts
QUANTITATIVE CHEMISTRY - GCSE Chemistry (AQA Topic C3)

Exphub 9th &10th
Class 10th Science - Complete Chemistry in One Shot🔥| Important Questions | Prashant Kirad
Summary
00:00
Balancing Chemical Equations with Precision
- To balance equations, start by drawing a line down the middle and counting atoms on each side. Begin with the metals, then nonmetals, and finally hydrogen or other elements. Adjust coefficients to balance the equation, ensuring the same number of each element on both sides.
- When balancing specific equations like iron and oxygen, identify the number of each element on both sides. Start with the metal, balance it by adjusting coefficients, then move to nonmetals like oxygen. Multiply the smallest common factor to balance the equation accurately.
- In the process of balancing equations like potassium, chlorine, and oxygen, carefully count and balance each element on both sides. Adjust coefficients for each element, ensuring the same number of each element on both sides to achieve a balanced equation.




