GCSE Chemistry - What is an Ionic Compound? Ionic Compounds Explained #15
Cognito・2 minutes read
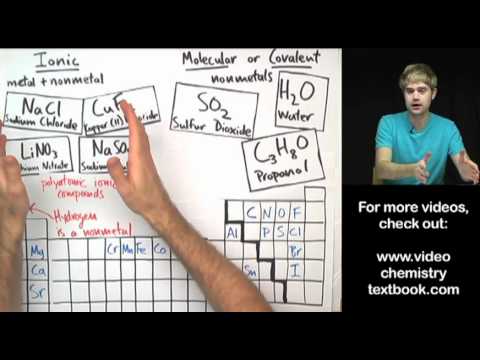
Ionic compounds are formed through ionic bonding with metal atoms transferring electrons to non-metal atoms, resulting in oppositely charged ions attracting each other. These compounds have high melting and boiling points, conduct electricity when melted or dissolved in water, and their formulas involve balancing charges of ions like NaCl and MgCl2, as well as memorizing common ion charges and formulas.
Insights
- Ionic compounds are created when a metal atom gives electrons to a non-metal atom, leading to charged ions that attract each other due to electrostatic forces, forming a structured lattice.
- The distinctive features of ionic compounds encompass their high melting and boiling points, along with the capacity to conduct electricity when dissolved or melted, facilitated by the movement of charged particles.
Get key ideas from YouTube videos. It’s free
Recent questions
How are ionic compounds formed?
Through ionic bonding, metal atoms transfer electrons to non-metal atoms.
Related videos
Summary
00:00
"Formation and Properties of Ionic Compounds"
- Ionic compounds are formed through ionic bonding, where a metal atom transfers electrons to a non-metal atom, resulting in oppositely charged ions that are attracted to each other by electrostatic forces, forming a three-dimensional regular lattice structure.
- Properties of ionic compounds include high melting and boiling points, as well as the ability to conduct electricity when melted or dissolved in water due to the movement of charged particles.
- Determining the formula of an ionic compound involves balancing the charges of the ions involved, such as in the case of sodium chloride (NaCl) or magnesium chloride (MgCl2), and memorizing the charges and formulas of common ions like hydroxide (OH-), sulfate (SO4 2-), nitrate (NO3-), carbonate (CO3 2-), and ammonium (NH4+).