ALL of Edexcel IGCSE Chemistry 9-1 | PAPER 1 / DOUBLE AWARD | IGCSE Chemistry Revision
Science with Hazel・93 minutes read
The video tutorial covers various chemistry topics like states of matter, diffusion, solutions, atoms, elements, periodic table, bonding, reactions, and practical experiments. It also delves into concepts such as oxidation, reduction, pH indicators, salts, and identifying metal ions through flame tests and precipitation reactions.
Insights
- The tutorial covers fundamental chemistry concepts like states of matter, diffusion, and solutions, providing a solid foundation for understanding more complex topics.
- Detailed explanations on the periodic table, group and period numbers, and chemical bonding help in comprehending the behavior and properties of elements.
- Various separation techniques and practical examples enhance understanding of how to isolate components from mixtures effectively.
- The text delves into practical applications of chemistry, such as calculating reacting masses, balancing equations, and identifying salts, offering real-world relevance to theoretical knowledge.
Get key ideas from YouTube videos. It’s free
Recent questions
What are the properties of noble gases?
Noble gases are unreactive due to full outer shells.
Related videos

LearnoHub - Class 11, 12
Some Basic Concepts Of Chemistry Class 11 Chemistry NCERT Chapter 1 #1 | Atharv Batch

Exam Winner SSLC
SSLC Chemistry | Reactivity Series and Electrochemistry |Full Chapter | Exam Winner
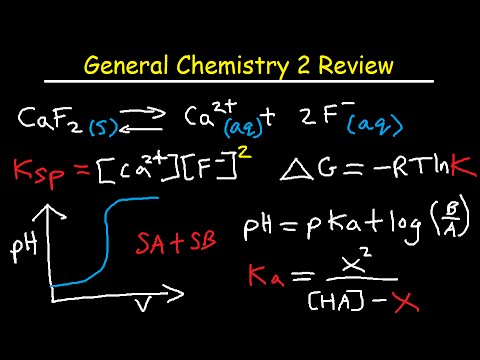
The Organic Chemistry Tutor
General Chemistry 2 Review Study Guide - IB, AP, & College Chem Final Exam

LearnoHub - Class 11, 12
States of Matter - Class 11 Chemistry | Chapter 5 | One Shot

Miss Natalie Chemistry
1AB Equations and Types of Reactions - Edexcel IAS Chemistry (Unit 1)
Summary
00:00
Chemistry Tutorial: States, Solutions, Separation Techniques
- The video is an Excel all-in-one chemistry guide for the 91 new specification, with revision guides available on the website www.hyken.com.
- The tutorial begins with an explanation of solids, liquids, and gases, detailing their particle arrangements and kinetic energy levels.
- Conversions between states of matter are discussed, such as melting, freezing, boiling, and condensation.
- Evaporation is explained as the process where particles with higher kinetic energy leave the liquid surface first.
- Diffusion is defined as the movement of particles from high to low concentration without energy input.
- An example of diffusion involving ammonia and hydrochloric acid is provided, showcasing the concept.
- The text delves into solutions, defining solute, solvent, solution, and saturated solution using coffee as an example.
- Fundamental chemistry concepts like atoms, elements, compounds, and mixtures are explained, with practical examples like making a cake.
- The distinction between pure substances, which have fixed boiling points, and mixtures, which boil over a range of temperatures, is highlighted.
- Various separation techniques like filtration, evaporation, distillation, and chromatography are detailed with practical examples and apparatus descriptions.
15:17
Chemical properties and bonding in elements
- Group numbers on the periodic table correspond to the number of electrons in the outer shell, with Group one elements having one electron in the outer shell.
- Period numbers on the periodic table refer to the number of electron shells, influencing the chemical properties of elements in the same group.
- Elements in the same group exhibit similar chemical properties due to having the same number of electrons in the outer shell.
- Noble gases in Group zero are unreactive because they have full outer shells, avoiding bonding.
- Metals have high melting and boiling points, conduct heat and electricity, are shiny, sonorous, malleable, and ductile.
- Metals tend to lose electrons in bonding to become positive ions, form basic oxides, and engage in ionic bonding.
- Nonmetals are dull, have low melting and boiling points, are brittle, form acidic oxides, gain electrons in bonding to become negative ions, and partake in covalent and ionic bonding.
- Ions are charged particles formed by gaining or losing electrons, altering their charge.
- Balancing chemical equations involves tallying elements on each side, adjusting with big numbers, and ensuring the same number of each element.
- Calculations using the formula triangle involve mass, relative atomic mass, and moles, aiding in determining molecular formulas and reacting masses.
31:17
Chemical Calculations and Bonding in Chemistry
- To find the number of moles, divide the mass by the molar mass, such as 3.2 divided by 63.5 to get 0.05 moles.
- For larger numbers, like 4, multiply the number of moles by the coefficient, so 0.05 times 4 equals 0.2 moles.
- Comparing the given amount with the required amount shows if a substance is in excess, like having 0.4 moles of nitric acid when only 0.26 moles are needed.
- Calculating percentage yield involves dividing the actual yield by the theoretical yield and multiplying by 100, as in 11.2 divided by 12.5 times 100 equals 89.6%.
- In a more complex example, balancing the equation for the reaction and calculating the theoretical yield using molar masses is crucial.
- Memorizing certain ions, like transition metals and negative ions combined with oxygen, is necessary for chemical formulas.
- Forming ionic compounds involves balancing charges, like needing two chlorines for one magnesium to create magnesium chloride.
- Understanding covalent bonding involves sharing electrons between nonmetals, as seen in water (H2O) and methane (CH4) structures.
- Explaining the formation of carbon dioxide (CO2) with double covalent bonds showcases the complexity of certain molecules.
- Learning about giant ionic structures, formed by metals and nonmetals with strong electrostatic forces, helps understand their high melting points and properties.
48:14
Ionic and Covalent Structures and Properties
- Solid ions do not conduct because they are not free to move, while molten or liquid ions conduct due to their free movement.
- Brittle materials smash easily when hit because the layers of ions slide upon impact, causing repulsion between like charges and breaking the structure.
- Diamond's high melting point is due to its giant tetrahedral structure with strong covalent bonds requiring significant energy to break.
- Graphite, with a slightly lower melting point than diamond, has a similar structure but each carbon atom is bonded to three others.
- Graphite is used as a lubricant due to weak intermolecular forces between layers, allowing easy sliding.
- Diamond does not conduct electricity as it lacks free electrons, while graphite does due to the presence of a free electron.
- C60 fullerene has a simple molecular structure with low melting point and does not conduct electricity due to the lack of free-moving electrons.
- Simple molecular substances have low melting points due to weak intermolecular forces that require minimal energy to break.
- Group one elements, alkali metals, are soft, reactive, and must be stored in oil to prevent reactions with moisture.
- Halogens, group seven elements, react with hydrogen to form hydrogen halides, are acidic and poisonous, and undergo displacement reactions based on reactivity.
01:03:17
Chemistry Fundamentals: Reactions and Properties
- Galvanizing involves using a more reactive metal like zinc to protect iron from rusting by donating electrons to the iron.
- Sacrificial protection is the term for this process where the more reactive metal reacts in preference to iron.
- Oxidation is the loss of electrons, while reduction is the gain of electrons, and redox reactions involve both processes simultaneously.
- Indicators like universal indicator show pH levels, with green for neutral, purple for alkali, and red for strong acid.
- Acids donate hydrogen ions, while alkalis donate hydroxide ions, with bases including metal carbonates, hydroxides, and oxides.
- Salts are formed when the hydrogen of an acid is replaced with a metal or ammonium, creating compounds like potassium chloride or calcium sulfate.
- Only metals above hydrogen in reactivity will react with acids, with highly reactive metals like potassium, sodium, and lithium reacting explosively.
- Specific salt equations detail reactions like metal + acid forming salt + hydrogen, or metal oxide + acid producing salt + water.
- Solubility rules dictate that nitrates, potassium, ammonium, and sodium compounds are soluble, while carbonates and hydroxides are generally insoluble.
- Flame tests and precipitation reactions help identify metal ions, with colors like red for lithium, yellow for sodium, and blue-green for copper.
01:17:43
"Thermodynamics Experiments: Acid-Base Reactions and Entropy"
- To create the potassium hydroxide solution, add 5 cm³ of nitric acid from the burette and stir the mixture.
- Measure the highest temperature reached after mixing and add another 5 cm³ of nitric acid, repeating the process.
- Use a pipette or burette to measure the 25 cm³ of potassium hydroxide solution accurately.
- Analyze the student's results, noting the highest temperature was reached with 30 cm³ of acid, while 20 cm³ showed an anomalous result.
- Possible mistakes causing the anomalous result include adding less than 5 cm³ of acid, not waiting for the highest temperature, or inadequate stirring.
- Suggest a temperature value for 120 cm³ of acid added, aiming for a value between 29 and 37.
- Calculate the heat energy released using the equation Q = MCΔT, considering the specific heat capacity and temperature change.
- Add 5 grams of excess zinc powder to 250 cm³ of 0.5 mol/dm³ copper sulfate solution in a glass beaker, noting initial and final temperatures.
- Calculate the energy change (Q) using Q = MCΔT, considering the mass of the liquids added.
- Determine the entropy change (ΔH) of the reaction using the equation ΔH = Q/N, calculating the number of moles and ensuring Q is in the correct unit.
01:32:50
"Hydrocarbons: Isomers, Fuels, and Distillation"
- Three carbon atoms form C3H6, representing an alkene, with a reminder to fill them up properly.
- Four carbon atoms create C4H8, known as butene, with the possibility of isomers due to different structural formulas.
- Isomers of C4H8 are named based on the location of the double bond, such as but-1-ene and but-2-ene.
- Exploring isomers for C5H12, identified as pentane, involves drawing straight chain and branched versions.
- Naming isomers like 2-methylbutane and 2,2-dimethylpropane follows the longest carbon chain and methyl group locations.
- Homologous series share chemical properties, functional groups, and gradual changes in physical properties.
- Alkenes and alkanes, derived from crude oil, serve as essential fuels due to their energy release when burnt.
- Fractional distillation of crude oil involves heating, vaporizing, and condensing different fractions based on boiling points.
- Various fractions like refinery gas, petrol, kerosene, diesel, fuel oil, and bitumen have distinct uses from central heating to road surfacing.
- Understanding viscosity, flammability, volatility, and cracking in hydrocarbons aids in comparing and utilizing different fractions effectively.




