LO3 - Chemical Energetics - Questions
Carboxyl・2 minutes read
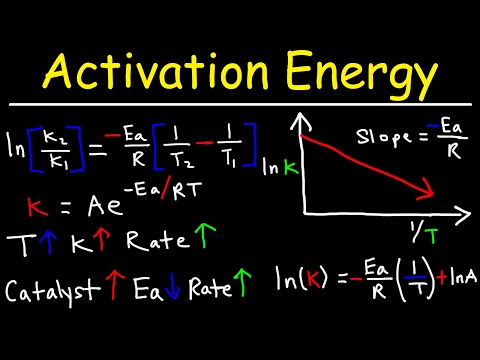
Nitrogen monoxide is an atmospheric pollutant causing irritation, formed in car engines through a reaction between nitrogen and oxygen. Understanding energy profiles, activation energy, and bond energy calculations are crucial in differentiating between endothermic and exothermic reactions, with catalysts playing a key role in speeding up reactions by lowering activation energy.
Insights
- Positive enthalpy change in a reaction signifies that more energy is needed to break bonds than is released during bond formation, indicating an endothermic process where products have higher energy levels than reactants.
- Catalysts play a crucial role in accelerating reactions by reducing the activation energy required for the reaction to occur, facilitating faster transformations without being consumed in the process.
Get key ideas from YouTube videos. It’s free
Recent questions
What is the role of activation energy in catalyzed reactions?
Activation energy in catalyzed reactions is lowered by catalysts, speeding up the reaction without being consumed.
How can one differentiate between endothermic and exothermic reactions?
Endothermic reactions absorb heat energy, while exothermic reactions release heat energy.
What is the significance of enthalpy change in chemical reactions?
Enthalpy change indicates the energy absorbed or released during bond formation and breaking.
How are energy profile diagrams used to analyze reactions?
Energy profile diagrams depict reactants, products, enthalpy change, and activation energy in reactions.
How do bond energy calculations contribute to understanding reactions?
Bond energy calculations involve determining the energy required to break and form specific bonds in a reaction.
Related videos

JR Tutorials
9th Science | Chapter 7 | Energy Flow in Ecosystem | Lecture 3 | maharashtra board |
The Organic Chemistry Tutor
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics

Sir Tarun Rupani
Mole Concept and Stoichiometry One Shot | Mole Concept ICSE Class 10 | @sirtarunrupani

DW Planet A
Can this magic fuel clean up the shipping industry?

UDAAN
Carbon And Its Compounds FULL CHAPTER | Class 10th Science | Chapter 04 | Udaan
Summary
00:00
Energy Changes in Chemical Reactions
- Starting the recording with MCQs and the third question
- Nitrogen monoxide as an atmospheric pollutant causing irritation
- Formed in car engines by nitrogen and oxygen reaction
- Positive enthalpy change indicates endothermic reaction
- Analyzing energy taken in and given out for endo and exo reactions
- Photosynthesis process involving carbon dioxide and water
- Activation energy and enthalpy change in catalyzed reactions
- Increase in particle energy in reactions due to temperature rise
- Differentiating between endo and exo reactions based on bond breaking and formation
- Understanding energy profile diagrams and activation energy for reactions
16:09
Understanding Enthalpy Change and Bond Energy
- Activation energy of the reverse reaction is +447 for the forward reaction and the reactant becomes the product in the reverse reaction.
- Activation energy is never negative as energy is a scalar quantity, and in enthalpy change, negative values are adjusted.
- Positive enthalpy change in a reaction indicates that the energy needed to break bonds is greater than the energy released during bond formation.
- In an endothermic reaction, the products are at a higher energy level than the reactants due to the heat energy absorbed.
- The energy profile diagram for reactions should include reactants, products, enthalpy change, and activation energy.
- The examiner may specify whether to label reactants or products in the energy profile diagram.
- Endothermic reactions involve more energy taken in during bond breaking than given out during bond formation.
- Bond energy calculations involve determining the energy required to break and form specific bonds in a reaction.
- Enthalpy change in a reaction can be calculated by subtracting the energy released from the energy absorbed during bond formation and breaking.
- The enthalpy change in a reaction can be used to calculate the bond energy of specific bonds, such as the bond between carbon and oxygen in carbon monoxide.
32:19
Bond Energy Shifts, Energy Calculations in Chemistry
- Bond energy cannot be negative; it shifts from negative to positive when a bond is formed and released.
- To calculate energy released during sulfur burning, convert moles to mass using molar mass.
- When one mole of sulfur burns, it releases 247 kJ of energy; calculate energy for 0.3 moles.
- Energy taken in during bond breaking is more than energy given out during bond formation.
- Calculate energy needed to break specific bonds by multiplying the number of bonds with their respective energies.
- Calculate total energy required to break one mole of iodine and chlorine bonds.
- Enthalpy change is calculated by subtracting energy given out during bond formation from energy taken in during bond breaking.
- Calculate energy released when hydrogen peroxide burns by converting moles to mass and using energy values.
- Catalysts speed up reactions by lowering activation energy without being consumed in the process.




