GCSE Physics Revision "Atomic Structure"
Freesciencelessons・3 minutes read
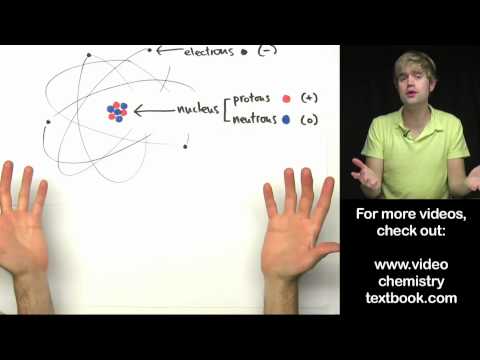
Atoms are extremely small, with a radius of about 1 × 10^-10 meters, where the nucleus holds protons and neutrons and is significantly smaller in comparison. Electrons reside in energy levels around the nucleus and can transition between these levels through the absorption or emission of electromagnetic radiation.
Insights
- Atoms are incredibly small, with a radius of about 1 × 10^-10 meters, and their nucleus is even tinier, containing protons that carry a positive charge and neutrons that have no charge, highlighting the fundamental structure of matter.
- Electrons, which are found at different distances from the nucleus, can shift between energy levels by absorbing or emitting electromagnetic radiation, illustrating how energy interactions play a crucial role in the behavior of atoms.
Get key ideas from YouTube videos. It’s free
Recent questions
What is an atom made of?
An atom consists of a nucleus and electrons. The nucleus contains protons, which have a positive charge, and neutrons, which are neutral. Surrounding the nucleus are electrons that occupy different energy levels. These components work together to define the structure and behavior of the atom, making it the fundamental building block of matter.
How do electrons move in an atom?
Electrons move between energy levels in an atom by absorbing or emitting electromagnetic radiation. When an electron gains energy, it can jump to a higher energy level, while losing energy allows it to fall back to a lower level. This movement is essential for understanding atomic interactions and the emission or absorption of light, which is a key concept in quantum mechanics.
What is the size of an atom?
Atoms have a radius of approximately 1 × 10^-10 meters, which is incredibly small. The nucleus of the atom, where protons and neutrons reside, is even smaller, measuring less than 1/1000th of the atom's total radius. This minuscule size highlights the vast empty space that exists within an atom, emphasizing the unique structure of matter at the atomic level.
What are protons and neutrons?
Protons and neutrons are subatomic particles found in the nucleus of an atom. Protons carry a positive charge, which contributes to the atom's overall charge and determines its identity as a specific element. Neutrons, on the other hand, are neutral and do not carry any charge. Together, these particles play a crucial role in the stability and properties of the atom, influencing its behavior in chemical reactions.
What is electromagnetic radiation?
Electromagnetic radiation is a form of energy that travels through space and can be absorbed or emitted by atoms. It encompasses a wide range of wavelengths, including visible light, radio waves, and X-rays. In the context of atomic structure, electromagnetic radiation is essential for the movement of electrons between energy levels, as it allows them to gain or lose energy, thus changing their state within the atom.
Related videos
Tyler DeWitt
Basic Atomic Structure: A Look Inside the Atom

PBS Space Time
Did AI Prove Our Proton Model WRONG?

Bryson Chemistry
Electron Shells, Sub-shells and Orbitals

Khan Academy
Introduction to the atom | Chemistry of life | Biology | Khan Academy

Garrison Student Hub
Structure of an atom ch#4 pg# 48 secondary Science class 7 explanation with Test yourself
Summary
00:00
Understanding Atomic Structure and Electron Behavior
- Atoms have a radius of approximately 1 × 10^-10 meters, with the nucleus being less than 1/1000th of this radius, containing protons (positive charge) and neutrons (neutral).
- Electrons occupy energy levels at varying distances from the nucleus; they can move between levels by absorbing or emitting electromagnetic radiation, changing their energy state.




