7A Intermolecular Forces - Edexcel IAS Chemistry (Unit 2)
Miss Natalie Chemistry・2 minutes read
Intermolecular forces, including London dispersion forces, permanent dipole-dipole interactions, and hydrogen bonds, are essential concepts in chemistry, with hydrogen bonds being the strongest and crucial for molecular interactions like those in water. Understanding the characteristics and examples of these forces, particularly the role of electronegativity and molecular geometry, is vital for analyzing chemical behavior and answering exam questions effectively.
Insights
- Intermolecular forces are essential interactions between molecules that differ from stronger covalent or ionic bonds, with three main types: London forces, permanent dipole-dipole interactions, and hydrogen bonds, the latter being the strongest and crucial for the properties of substances like water, which showcases the importance of O-H groups in forming these bonds.
- Understanding the nuances of these forces is vital for analyzing molecules, as seen in glucose, where both London forces and permanent dipole-dipole interactions due to O-H groups play significant roles; recognizing these forces and the specific atoms involved is key in academic assessments, emphasizing the need for clarity in identifying types of interactions and their contributing atoms.
Get key ideas from YouTube videos. It’s free
Recent questions
What are intermolecular forces?
Intermolecular forces are the interactions that occur between molecules, distinct from the stronger covalent, ionic, and metallic bonds that hold atoms together within a molecule. These forces are generally weaker than the aforementioned types of bonding and play a crucial role in determining the physical properties of substances, such as boiling and melting points. The three primary types of intermolecular forces include London dispersion forces, permanent dipole-dipole interactions, and hydrogen bonds. Understanding these forces is essential for grasping how different substances behave in various conditions, as they influence the arrangement and movement of molecules in a given material.
How do London forces work?
London forces, also known as London dispersion forces, are a type of intermolecular force that arises from the movement of electrons within molecules. These forces exist in all substances that contain electrons and occur due to the creation of instantaneous dipoles, which are temporary shifts in electron density that can induce dipoles in neighboring molecules. The strength of London forces is influenced by the number of electrons in a molecule and the surface area available for contact; larger molecules with more electrons and greater surface area exhibit stronger London forces. This phenomenon explains why substances like butane, which has a longer carbon chain, can experience enhanced London forces compared to smaller molecules.
What are hydrogen bonds?
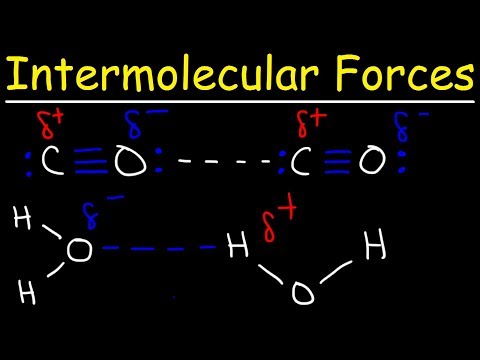
Hydrogen bonds are a specific type of strong intermolecular force that occurs when hydrogen is covalently bonded to highly electronegative atoms such as fluorine, oxygen, or nitrogen. This bonding creates a significant dipole, where the hydrogen atom carries a partial positive charge, while the electronegative atom carries a partial negative charge. The attraction between these opposite charges leads to the formation of hydrogen bonds, which are notably stronger than other types of intermolecular forces, such as London forces and permanent dipole-dipole interactions. A common example of hydrogen bonding can be observed in water molecules, where the O-H groups facilitate strong attractions between adjacent water molecules, significantly influencing water's unique physical properties.
What is a permanent dipole?
A permanent dipole occurs in molecules where there is a significant difference in electronegativity between the atoms involved in a bond, resulting in a consistent separation of charge. This creates a dipole moment, where one end of the molecule is partially positive and the other end is partially negative. Permanent dipole-dipole interactions arise when these dipoles attract or repel each other, influencing the physical properties of substances. However, these interactions are generally weaker than hydrogen bonds and can be less significant than London forces, depending on the molecular structure and the presence of other types of intermolecular forces. Understanding permanent dipoles is essential for predicting how molecules will interact in various chemical and physical contexts.
How do intermolecular forces affect boiling points?
Intermolecular forces play a critical role in determining the boiling points of substances. The strength of these forces influences how much energy is required to separate molecules from one another during the phase transition from liquid to gas. Substances with stronger intermolecular forces, such as hydrogen bonds, typically have higher boiling points because more energy is needed to overcome these attractions. Conversely, substances that primarily exhibit weaker London forces will generally have lower boiling points, as less energy is required to separate the molecules. Therefore, understanding the types and strengths of intermolecular forces present in a substance is essential for predicting its boiling point and other physical properties.
Related videos

The Organic Chemistry Tutor
Intermolecular Forces - Hydrogen Bonding, Dipole-Dipole, Ion-Dipole, London Dispersion Interactions
The Organic Chemistry Tutor
Intermolecular Forces - Hydrogen Bonding, Dipole Dipole Interactions - Boiling Point & Solubility

Allery Chemistry
EDEXCEL Topic 2 Bonding and Structure REVISION

Arjuna JEE
Chemical Bonding: COMPLETE Chapter in 1 Video | Quick Revision | Class 11 Arjuna JEE

Thomas Mennella
Lecture 1A: Water (Polarity)
Summary
00:00
Understanding Intermolecular Forces and Their Types
- Intermolecular forces, also known as non-bonded forces, are interactions between molecules, distinct from covalent, ionic, and metallic bonds, and are generally weaker than these types of bonding.
- The three main types of intermolecular forces to understand are London forces (or London dispersion forces), permanent dipole-dipole interactions, and hydrogen bonds, with the latter being the strongest.
- London forces exist in all substances with electrons; they arise from the movement of electrons creating instantaneous dipoles due to unsymmetrical electron density, which can induce dipoles in neighboring molecules.
- The strength of London forces increases with the number of electrons in a molecule and the surface area available for contact; for example, butane's longer chain allows for more points of contact, enhancing London forces.
- Permanent dipole-dipole interactions occur when there is a significant difference in electronegativity between atoms in a molecule, resulting in a consistent dipole that can attract or repel other permanent dipoles, but these interactions are less significant than London forces due to potential repulsion.
- Hydrogen bonds form when hydrogen is covalently bonded to highly electronegative atoms like fluorine, oxygen, or nitrogen, creating strong intermolecular attractions; for instance, water molecules exhibit hydrogen bonding due to the presence of O-H groups.
- The bond angle in hydrogen bonds is always 180 degrees, indicating a linear arrangement, which is crucial for understanding molecular geometry in questions related to intermolecular forces.
- Examples of substances that can form hydrogen bonds include water (H2O), alcohols, and carboxylic acids, all containing O-H groups, while hydrogen fluoride (HF) is the only example with hydrogen bonded to fluorine.
- When analyzing glucose molecules, one should identify London forces due to the presence of electrons, as well as permanent dipole-dipole interactions from the O-H groups, which create regions of differing electronegativity.
- In exam questions regarding intermolecular forces, it is essential to recognize that London forces are always present, and to specify the types of forces and the atoms involved without needing to explain their formation unless explicitly asked.
20:04
Understanding Chemical Bond Formation and Forces
- The text discusses the formation of various types of chemical bonds, specifically highlighting that carbon can be Delta positive while oxygen is Delta negative, or hydrogen can be Delta positive with oxygen being Delta negative; it emphasizes the importance of specifying the atoms involved in these bonds, such as the hydrogen in the hydroxyl (OH) group bonding with another oxygen, and notes that one mark is awarded for correctly naming the forces and another mark for identifying the atoms involved; it also mentions that hydrogen bonds occur due to hydrogen being bonded to a highly electronegative element, and encourages viewers to check out related content on physical properties in topic 7b, inviting questions and comments for further clarification.




