What is Heat?, Temperature | Class 7 Science Chapter 3 | Heat
Magnet Brains・12 minutes read
HIT, a form of energy, transfers between bodies until temperature equilibrium is achieved, flowing from hot to cold objects regardless of size. Temperature, quantified using thermometers, measures heat within a body accurately in Celsius, Fahrenheit, or Kelvin.
Insights
- Heat (HIT) is a form of energy that moves from hot to cold objects until temperature equilibrium is reached, transferring through conduction, convection, and radiation.
- Temperature, quantified using thermometers in Celsius, Fahrenheit, or Kelvin, measures the hotness or coldness of a body accurately, with heat energy flowing until equilibrium is achieved.
Get key ideas from YouTube videos. It’s free
Recent questions
What is HIT energy?
HIT energy is a form of energy that is felt but not seen, similar to sunlight or the energy of hearing and seeing. It causes a surge of energy after eating, leading to sensations of warmth or nausea.
How does HIT energy transfer occur?
HIT energy transfer happens between bodies with a temperature difference, moving from high to low temperature until equilibrium is reached. It flows from hot to cold objects in all states - liquid, solid, and gas - through conduction, convection, and radiation.
What is the role of temperature in HIT energy transfer?
Temperature is the measurement of hotness or coldness, typically quantified in degrees Celsius, Fahrenheit, or Kelvin. It is crucial in HIT energy transfer as the energy flows until both bodies reach temperature equilibrium, with thermometers used to determine the exact heat value within a body.
How does the size of objects affect HIT energy transfer?
HIT energy transfer does not depend on the size of objects but continues until temperature equilibrium is achieved. The energy flows from hot to cold objects regardless of their size, emphasizing the importance of temperature difference in the process.
Why are thermometers essential in measuring temperature accurately?
Thermometers play a vital role in accurately measuring temperature, providing precise readings of hotness or coldness. They are crucial in determining the exact value of heat within a body, aiding in understanding the flow of HIT energy and achieving temperature equilibrium.
Related videos

Akshata Kirpekar
Concept of Latent Heat | Chapter 5 Heat | Class 10th Maharashtra State Board

Turtlediary
Heat Energy & How We Use It *COOL* Science for Kids!

CPPMechEngTutorials
Heat Transfer (02): Introductory examples, energy balance on a control volume and control surface

khanacademymedicine
Absolute temperature and the kelvin scale | Physical Processes | MCAT | Khan Academy
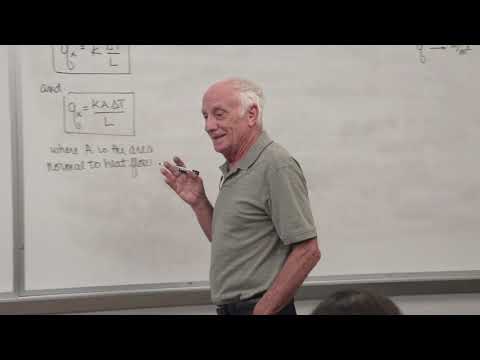
CPPMechEngTutorials
Heat Transfer (01): Introduction to heat transfer, conduction, convection, and radiation
Summary
00:00
Understanding HIT Energy Transfer Through Temperature
- HIT is a form of energy, akin to sunlight or the energy of hearing and seeing, which is only felt.
- HIT is an energy that causes a surge of energy after eating, leading to a feeling of warmth or nausea.
- HIT is transferred between bodies when there is a temperature difference, moving from high to low temperature until equilibrium is reached.
- The transfer of HIT continues until the temperature of both bodies is equal, with the energy flowing from hot to cold objects.
- HIT energy transfer occurs in all states - liquid, solid, and gas - through conduction, convection, and radiation.
- HIT energy does not depend on the size of objects but flows until temperature equilibrium is achieved.
- Temperature is the measurement of hotness or coldness, typically measured in degrees Celsius, Fahrenheit, or Kelvin.
- Temperature is quantified using a thermometer to determine the exact value of heat within a body.
- Thermometers are essential for measuring temperature accurately, providing precise readings of hotness or coldness.




