Chapter 13_Part1
Jeanne L. Bolliger・34 minutes read
Conjugated pi systems, such as 1,3-butadiene, feature alternating double and single bonds that allow for resonance stabilization and lower energy states, with molecular orbitals indicating charge distribution. UV spectroscopy reveals that increased conjugation in compounds like beta-carotene leads to specific light absorption characteristics, demonstrating the relationship between molecular structure and color in organic compounds.
Insights
- Conjugated pi systems, such as 1,3-butadiene, consist of alternating double and single bonds with sp2 hybridized, planar carbons, allowing for resonance stabilization; this stabilization lowers the overall energy of the molecule and enhances its stability, particularly when the resonance structures maintain full valence shells and minimal charge separation.
- The ability of conjugated systems to absorb UV light is linked to their molecular structure, as seen in compounds like beta-carotene, which has multiple double bonds that reduce the energy required for electron excitation; this results in visible color changes, demonstrating that greater conjugation corresponds to lower energy transitions and specific light absorption characteristics.
Get key ideas from YouTube videos. It’s free
Recent questions
What is a conjugated system?
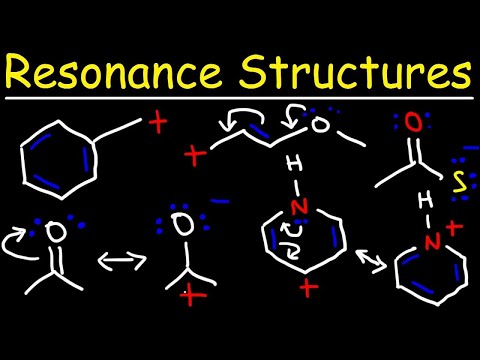
A conjugated system refers to a molecular structure characterized by alternating single and double bonds, which allows for the delocalization of electrons across the involved atoms. This arrangement typically involves sp2 hybridized carbons that are planar, enabling effective overlap of p orbitals. A classic example of a conjugated system is 1,3-butadiene, where the alternating double and single bonds create a stable configuration that can participate in resonance. The presence of conjugated systems is significant in various chemical properties, including stability and reactivity, as they can lower the overall energy of the molecule through resonance stabilization.
How does resonance stabilization work?
Resonance stabilization is a phenomenon where a molecule can be represented by multiple valid Lewis structures, known as resonance structures. These structures differ only in the arrangement of electrons, particularly in pi bonds or lone pairs, while the positions of the atoms remain unchanged. The actual molecule is a hybrid of these structures, leading to a lower overall energy state compared to any individual resonance form. Stability is enhanced when resonance structures maintain full valence shells and minimize charge separation. The delocalization of electrons across multiple atoms allows for greater stability, as seen in structures like the allyl cation, where charge is distributed over terminal carbons.
What is the significance of UV spectroscopy?
UV spectroscopy is a powerful analytical technique used to measure the absorption of ultraviolet and visible light by molecules, providing insights into their electronic structure. This method is particularly useful for identifying conjugated systems, as these structures absorb light at specific wavelengths due to electron transitions between molecular orbitals. The energy required for these transitions can be quantified, allowing chemists to determine the extent of conjugation in a molecule. For instance, compounds like butadiene and beta-carotene exhibit distinct absorption patterns that correlate with their conjugated double bonds, revealing their chemical properties and potential applications in fields such as biochemistry and materials science.
What are resonance structures?
Resonance structures are different Lewis structures that represent the same molecule, differing only in the distribution of electrons, particularly in pi bonds and lone pairs. These structures are essential for understanding the behavior of molecules that cannot be accurately depicted by a single Lewis structure. For a set of resonance structures to be valid, they must adhere to specific rules, such as maintaining the same number of unpaired electrons and ensuring that formal charges are correctly assigned. The actual molecule is a resonance hybrid, where the true electronic structure is a blend of all contributing forms, leading to enhanced stability and lower energy due to electron delocalization.
How do conjugated systems affect color?
Conjugated systems significantly influence the color of molecules due to their ability to absorb specific wavelengths of light. The presence of alternating double bonds allows for electron delocalization, which lowers the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). As a result, these molecules can absorb light in the ultraviolet or visible spectrum, leading to color changes. For example, carotenoids like beta-carotene, which contain multiple conjugated double bonds, absorb light at specific wavelengths, resulting in their characteristic colors. The relationship between absorbed and transmitted light can be visualized using a color wheel, where the absorbed color is complementary to the color observed.
Related videos

Najam Academy
Resonance Structures | How to draw resonant structures? Easy Trick
The Organic Chemistry Tutor
Resonance Structures

MJD Chemistry
Atomic orbital treatment of Benzene | ch#9 | 12th class chemistry
Khan Academy
Representing structures of organic molecules | Biology | Khan Academy

uma rathore
BSC lll paper lll MOT, valence bond treatment of hydrogen molecule
Summary
00:00
Understanding Conjugated Pi Systems and Resonance
- Conjugated pi systems consist of alternating double and single bonds, exemplified by 1,3-butadiene, where all involved carbons are sp2 hybridized and planar.
- The conjugated system can include heteroatoms, such as oxygen, maintaining the alternating double-single bond pattern, as seen in carbonyl compounds.
- Larger conjugated systems, like beta-carotene, can contain multiple alternating double bonds, totaling 11 in this case, and remain planar throughout.
- Non-conjugated unsaturated systems, like cumulenes, feature sp hybridized carbons that disrupt planarity, preventing resonance stabilization and conjugation.
- Isolated double bonds occur when sp3 hybridized carbons separate double bonds, breaking the alternating pattern necessary for conjugation, as seen in certain ketones.
- Allyl radicals are resonance-stabilized, allowing for electron delocalization across terminal carbons, with molecular orbitals showing bonding, nonbonding, and antibonding characteristics.
- The allyl cation, formed from an allylic leaving group, exhibits resonance stabilization, distributing charge across terminal carbons while maintaining a non-charged central carbon.
- Molecular orbitals for the allyl cation include a highest occupied molecular orbital (HOMO) and a lowest unoccupied molecular orbital (LUMO), with charge localized on terminal carbons.
- Resonance structures involve only the movement of electrons in pi bonds or lone pairs, adhering to proper Lewis structure rules, including maximum eight electrons for second-row elements.
- Proper resonance structures must maintain correct formal charges and avoid unrealistic configurations, ensuring they represent valid molecular forms.
21:21
Understanding Resonance Structures and Stability
- All resonance structures must maintain the same number of unpaired electrons; if one structure has a radical, all must also have a radical.
- A delocalized pi system in resonance structures must be planar or nearly planar to allow interaction between pi orbitals; perpendicular orbitals cannot interact.
- Resonance stabilization lowers the overall energy of a molecule; not all resonance structures contribute equally to the hybrid structure.
- The stability of resonance structures can be judged by counting covalent bonds; more covalent bonds indicate greater stability.
- Resonance structures with atoms lacking a full valence shell (e.g., six electrons on carbon) are less stable than those with full valence shells (eight electrons).
- Charge separation in resonance structures decreases stability; structures without formal charges are more stable than those with charge separation.
- In a conjugated ketone example, shifting double bond electrons creates resonance structures with positive and negative charges, affecting stability.
- One 3-butadiene is a planar conjugated system with sp2 hybridized carbons, resulting in shorter bond lengths compared to typical single bonds.
- The bond length of the central carbon-carbon single bond in one 3-butadiene is 1.47 angstroms, shorter than the 1.54 angstroms of a typical sp3-sp3 bond.
- UV spectroscopy measures energy absorption in the UV and visible range, indicating the presence of conjugated systems through color changes.
43:08
The Impact of UV Radiation on Molecules
- Ultraviolet (UV) radiation has short wavelengths and high frequencies, leading to high energy that can cause mutations; visible light ranges from approximately 400 to 700 nanometers.
- Molecules absorb light at specific wavelengths, with the absorbed color being complementary to the transmitted color; this relationship can be visualized using a color wheel.
- Butadiene has four pi electrons, with a homolumo gap that requires UV radiation for electron excitation; the energy needed for this transition can be measured using UV spectroscopy.
- Conjugation in molecules like butadiene and hexatriene decreases the homolumo gap, allowing for lower energy transitions; hexatriene requires 274 nanometers for excitation, easily achievable in a lab.
- Carotenoids like beta-carotene and lycopene, with 11 double bonds, absorb light at 477 and 505 nanometers respectively, demonstrating that increased conjugation leads to visible colors in molecules.




