4.2 Group No & Charge on an Ion | Chap 4 Periodic Table | class 9 Chemistry federal board NBF 2024
Federal Ka Manjan・20 minutes read
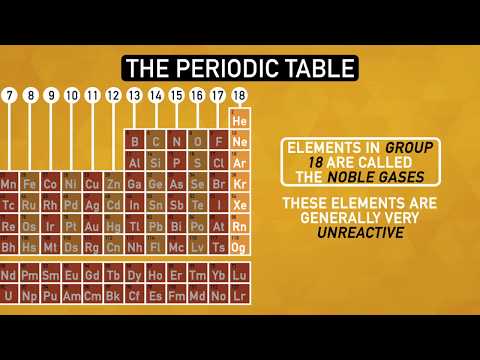
The lecture emphasizes the significance of understanding concepts without assuming conclusions, focusing on the development of basic elements to the periodic table and stability in atoms and molecules. Noble gases in group 18A are explained as stable due to electronic configuration and valence shell completion, with varying reactivity levels among different groups of elements, showcasing the complexity of chemical stability and reactions.
Insights
- Understanding stability in atoms and molecules is crucial in chemistry, with noble gases in group 18A serving as examples of stability due to their complete valence shells and low energy levels.
- The lecture stresses the importance of careful listening and avoiding premature conclusions, highlighting the need for thorough research and study to grasp fundamental concepts in chemistry, such as the behavior of elements based on electron interactions and valence shell completion.
Get key ideas from YouTube videos. It’s free
Recent questions
What is the significance of the periodic table in chemistry?
The periodic table is crucial in understanding element behavior and reactivity. It organizes elements based on their atomic number, electron configuration, and chemical properties. By following patterns within the table, scientists can predict how elements will interact with each other and form compounds. This organization helps in studying the stability of atoms and molecules, guiding research and experimentation in the field of chemistry.
How do noble gases differ from other elements?
Noble gases, found in group 18A of the periodic table, are known for their stability and low reactivity. This is due to their complete valence electron shells, making them less likely to form chemical bonds with other elements. Unlike other groups that readily give or take electrons to achieve stability, noble gases already have a full set of electrons, making them inert. Understanding the unique properties of noble gases is essential in comprehending the behavior of elements and the concept of stability in chemistry.
Why is stability important in atoms and molecules?
Stability in atoms and molecules is crucial for their existence and behavior. Just like humans seek stability in life, atoms and molecules strive to achieve the lowest energy state possible to maintain stability. This desire for stability influences how elements interact with each other, form compounds, and exhibit reactivity. By studying stability in chemistry, scientists can gain insights into the fundamental principles governing the behavior of matter at the atomic and molecular levels.
How do elements in different groups exhibit varying reactivity levels?
Elements in different groups of the periodic table display varying levels of reactivity based on their electron configurations and valence shell properties. For example, Alkaline Earth Metals in group 2 are capable of forming Plus 2 reactions by giving away two electrons, while elements in the 17th group can give two electrons to achieve stability. Understanding these group behaviors is essential in predicting how elements will interact with each other and form compounds, shedding light on the reactivity patterns observed in chemistry.
Can noble gases react under certain conditions?
While noble gases are generally stable and less reactive due to their complete valence electron shells, they can react under specific circumstances. Despite their inert nature, noble gases can form compounds if presented with opportunities to lower their energy levels further than naturally possible. The discovery of Krypton compounds after years of research highlights that even noble gases, known for their stability, can exhibit reactivity under certain conditions. This phenomenon challenges traditional notions of inertness and expands our understanding of element behavior in chemistry.
Related videos

Sourabh Raina
Classification of elements |20 Important questions |Class 11 Chemistry|Sourabh Raina

Math and Science
What is the Periodic Table? How are Elements Organized?
American Chemical Society
How The Periodic Table Organizes the Elements | Chemistry Basics

Unacademy NEET
Periodic Properties | Part 1 | Effective Nuclear Charge and Trends | NEET 2024 | Akansha Karnwal

Arjuna JEE
Chemical Bonding: COMPLETE Chapter in 1 Video | Quick Revision | Class 11 Arjuna JEE
Summary
00:00
Understanding Group Behavior and Charge in Chemistry
- Lecture on group number and charge on an account by Kartar Kumar on Fital Ka Manjan channel
- Emphasis on understanding concepts without jumping to conclusions
- Importance of not assuming conclusions and listening carefully to the lecture
- Focus on the development of concepts from basic elements to periodic table
- Significance of periodic table in understanding element behavior and reactivity
- Need for stability in atoms and molecules similar to human desire for stability in life
- Research and study required to understand stability in chemistry
- Introduction to noble gases in group 18A as stable and less reactive
- Explanation of stability based on electronic configuration and valence shell completion
- Detailed breakdown of group behaviors in terms of electron giving and taking, resulting in charges and stability levels
16:55
"Chemical Stability and Social Challenges Explored"
- Stability is defined by the least energy an atom or molecule possesses, with noble gases having the lowest energy and thus being stable.
- Noble gases, though generally stable, can react if presented with an opportunity to lower their energy further than naturally possible.
- Krypton compounds were discovered after several years, indicating that even noble gases can react under certain conditions.
- Different groups of elements have varying reactivity levels, with Alkaline Earth Metals capable of Plus 2 reactions and the 17th group able to give two electrons.
- Corruption and injustice in Pakistan are highlighted, showcasing the challenges faced within the country despite its Islamic foundation.
- A story of a Maulvi facing a dilemma due to threats from thieves illustrates the complexities and moral dilemmas individuals may encounter.
- The story of the Indian medical test results emphasizes the intense competition and challenges faced by students, with only the top 2000 out of thousands being selected as medical doctors.




